Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19
Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection (version 2.0) June 17th, 2020
Hydroxychloroquine and Chloroquine
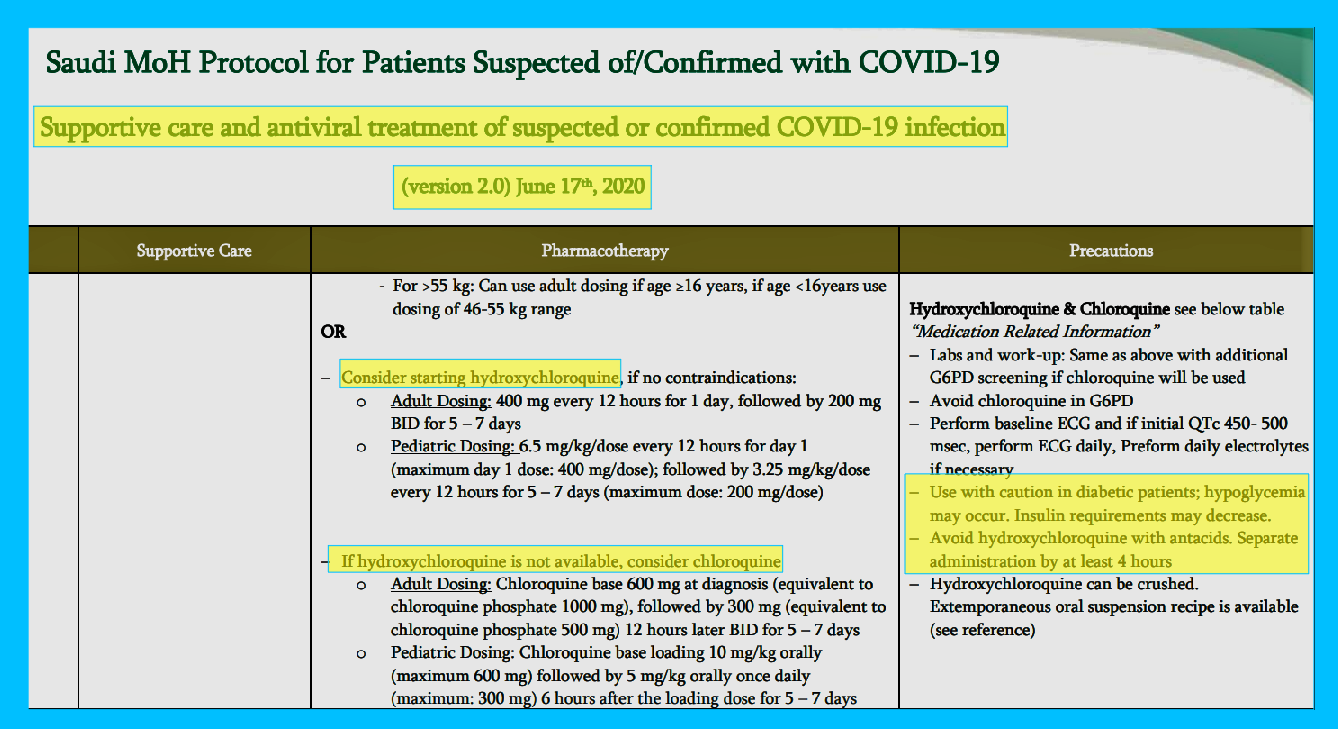
“Consider starting hydroxychloroquine, if no contraindications:
Adult Dosing: 400 mg every 12 hours for 1 day, followed by 200 mg
BID for 5 – 7 days Pediatric Dosing: 6.5 mg/kg/dose every 12 hours or day 1 (maximum day 1 dose: 400 mg/dose); followed by 3.25g/kg/dose every 12 hours for 5 – 7 days (maximum dose: 200 mg/dose)”
“If hydroxychloroquine is not available, consider chloroquine
Adult Dosing: Chloroquine base 600 mg at diagnosis (equivalent to
chloroquine phosphate 1000 mg), followed by 300 mg (equivalent to
chloroquine phosphate 500 mg) 12 hours later BID for 5 – 7 days Pediatric Dosing: Chloroquine base loading 10 mg/kg orally
(maximum 600 mg) followed by 5 mg/kg orally once daily
(maximum: 300 mg) 6 hours after the loading dose for 5 – 7 days”
Major Drug Interactions
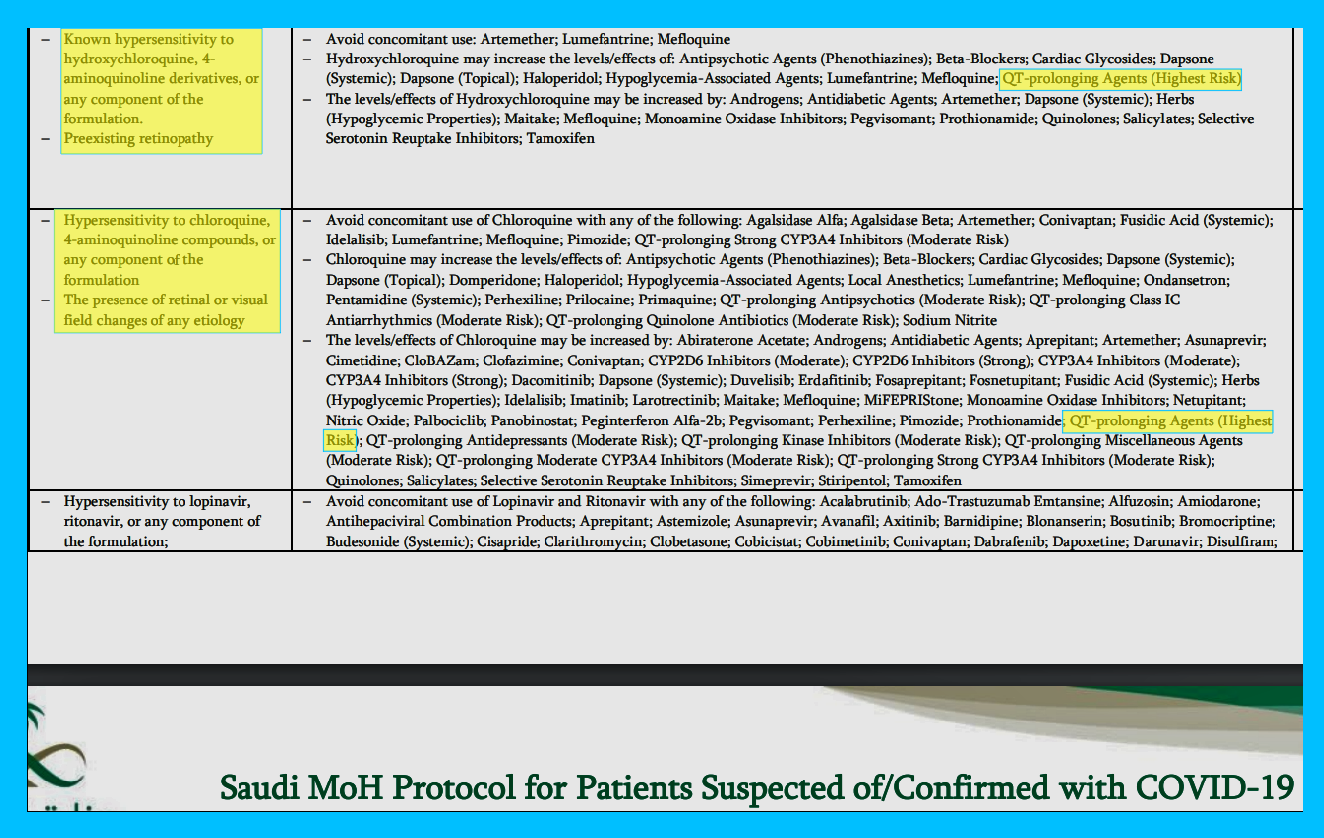
Hydroxychloroquine has fewer drug interaction warnings than Chloroquine, but the only one listed on both as being “Highest Risk” is the “QT-prolonging Agents”

Now compare the major drug interactions of Hydroxychloroquine and Chloroquine to Lopinavir/Ritonavir. There are five times the amount of major drug interactions and they even have one more “Highest Risk” the HCQ and CHQ: CYP3A4 Substrates & QT-prolonging Agents (Highest Risk). There approximately 500 major drug interactions associated with Lopinavir/Ritonavir.
KALETRA (lopinavir and ritonavir) tablet, for oral use
KALETRA (lopinavir and ritonavir) oral solution
Initial U.S. Approval: 2000
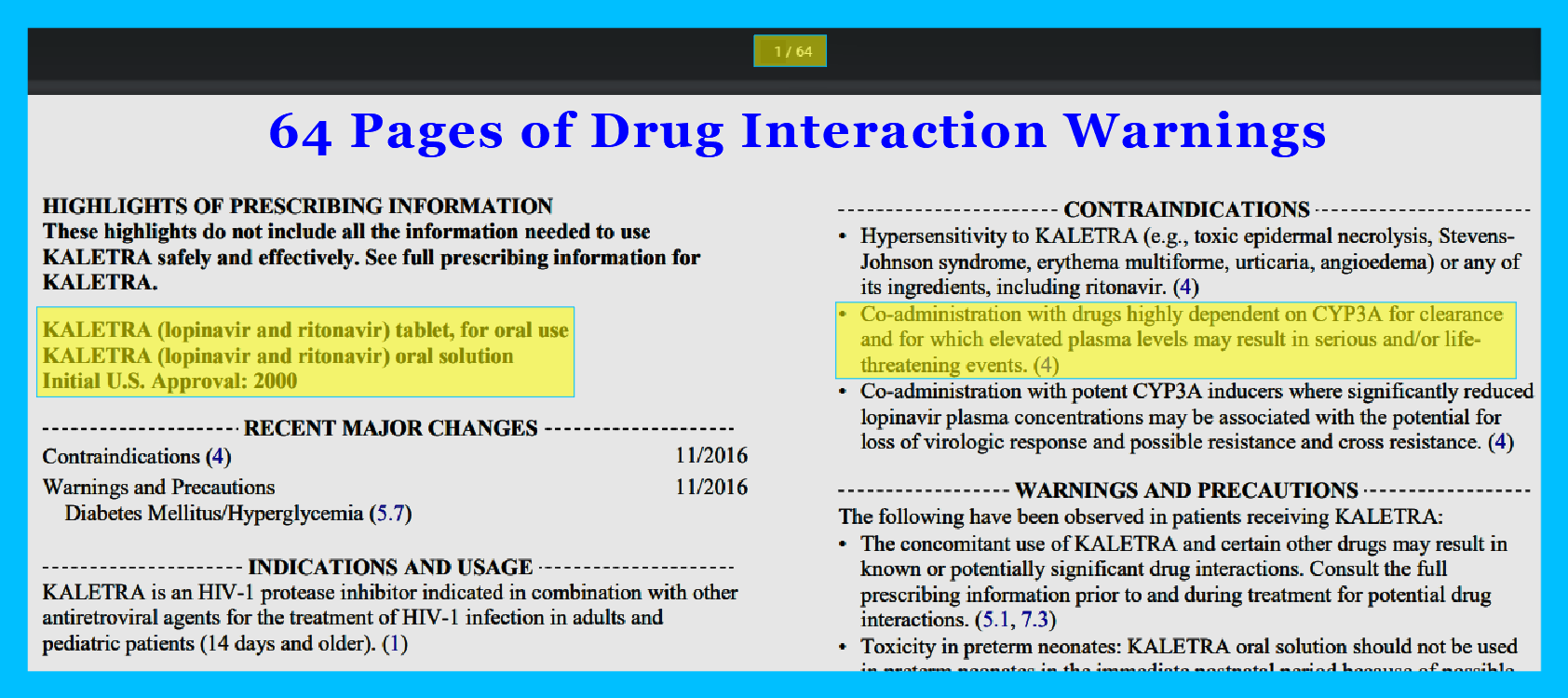
Lopinavir/Ritonavir (Kaletra) was approved by the FDA in 2000. The prescription label is 64 pages long to cover all the side effects and drug interactions.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf
PLAQUENIL® HYDROXYCHLOROQUINE SULFATE TABLETS, USP
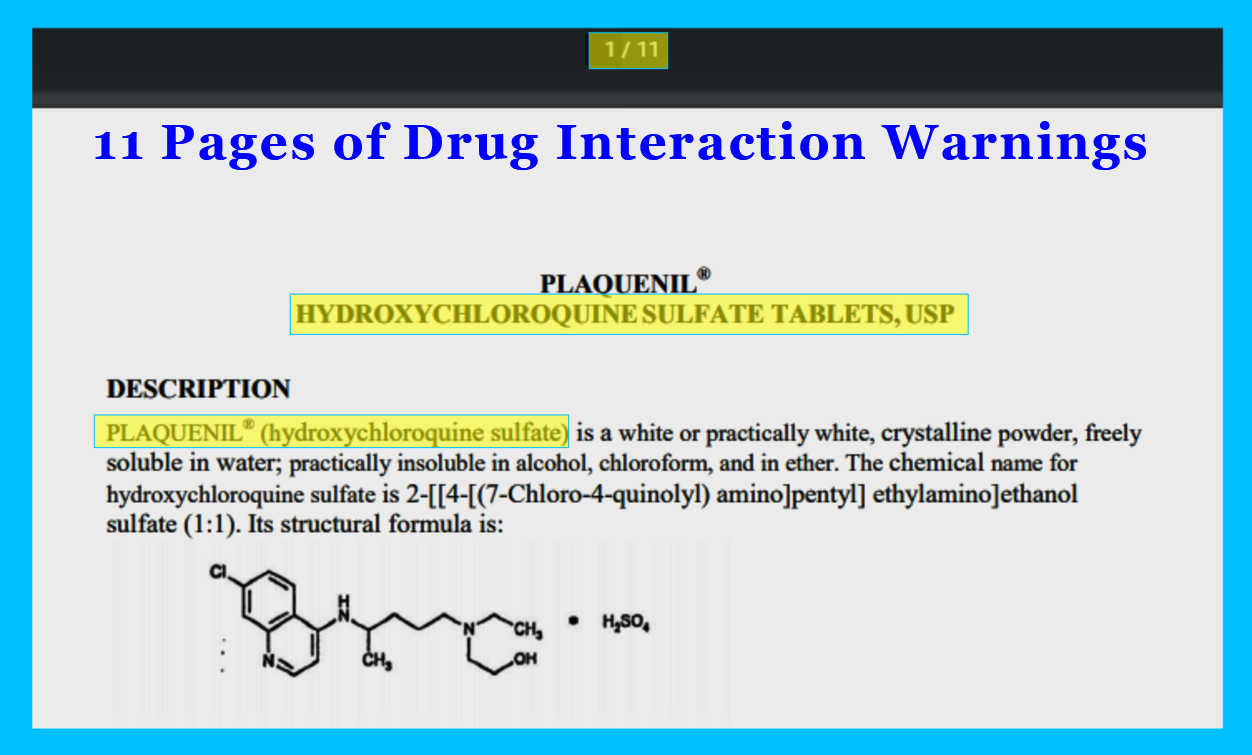
By comparison, the FDA prescription label for Hydroxychloroquine only has 11 pages of warnings and drug interactions.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf
ARALEN® CHLOROQUINE PHOSPHATE, USP
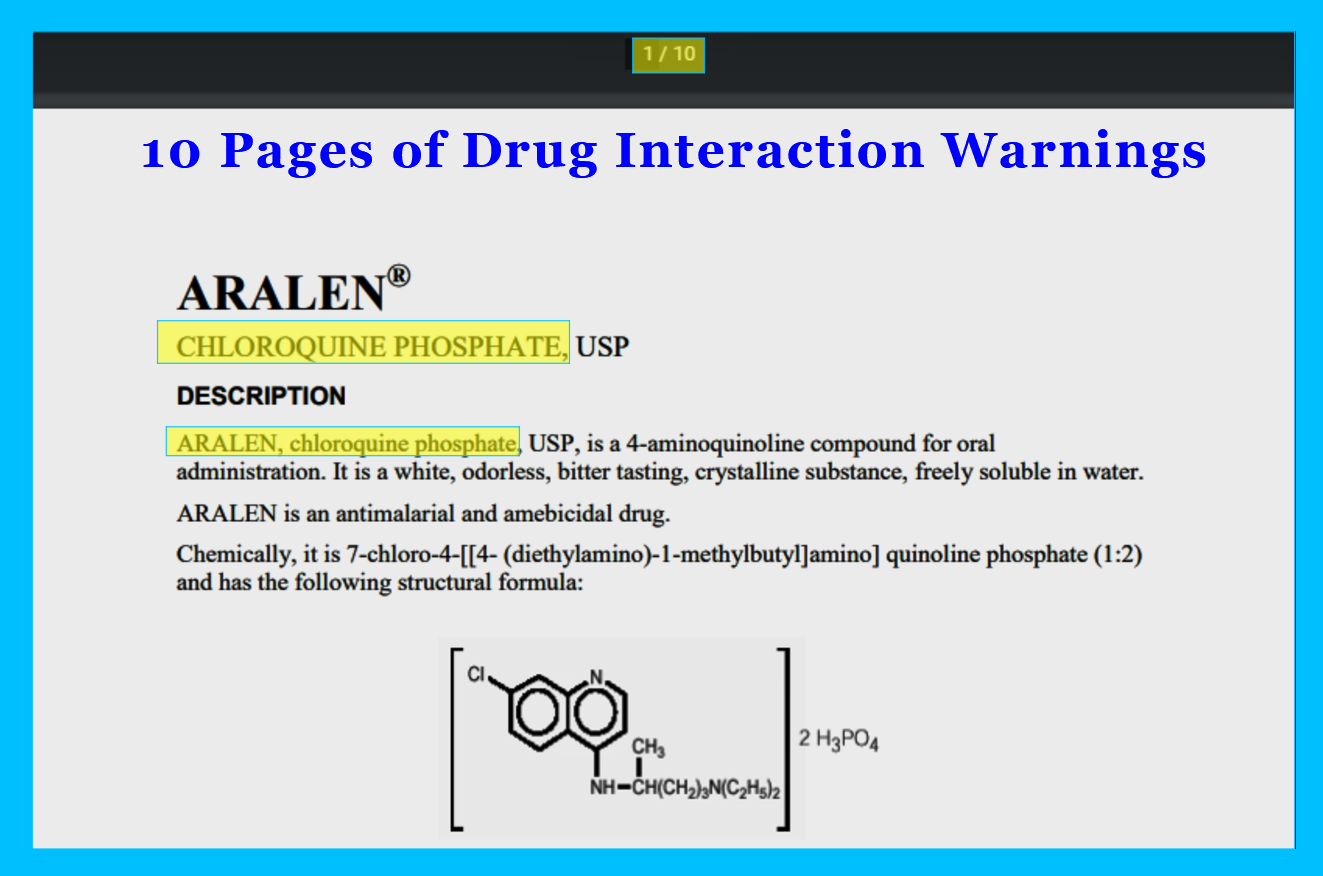
And Chloroquine only has 10 pages of warnings and drug interactions.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/006002s044lbl.pdf
So why does the FDA approve such a high risk drug with so many potential side effects and major drug interactions? (That question is rhetorical, we know why)
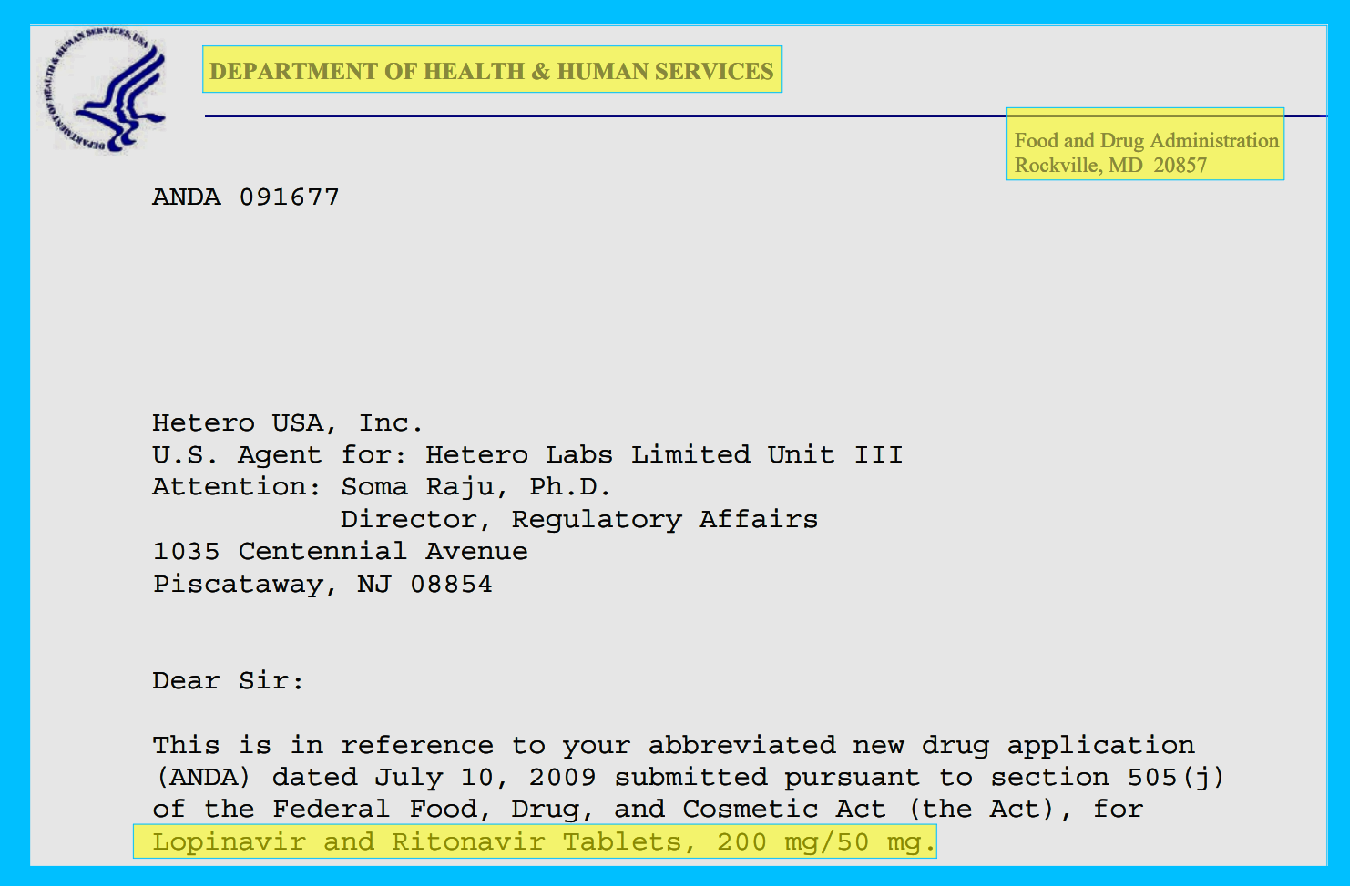
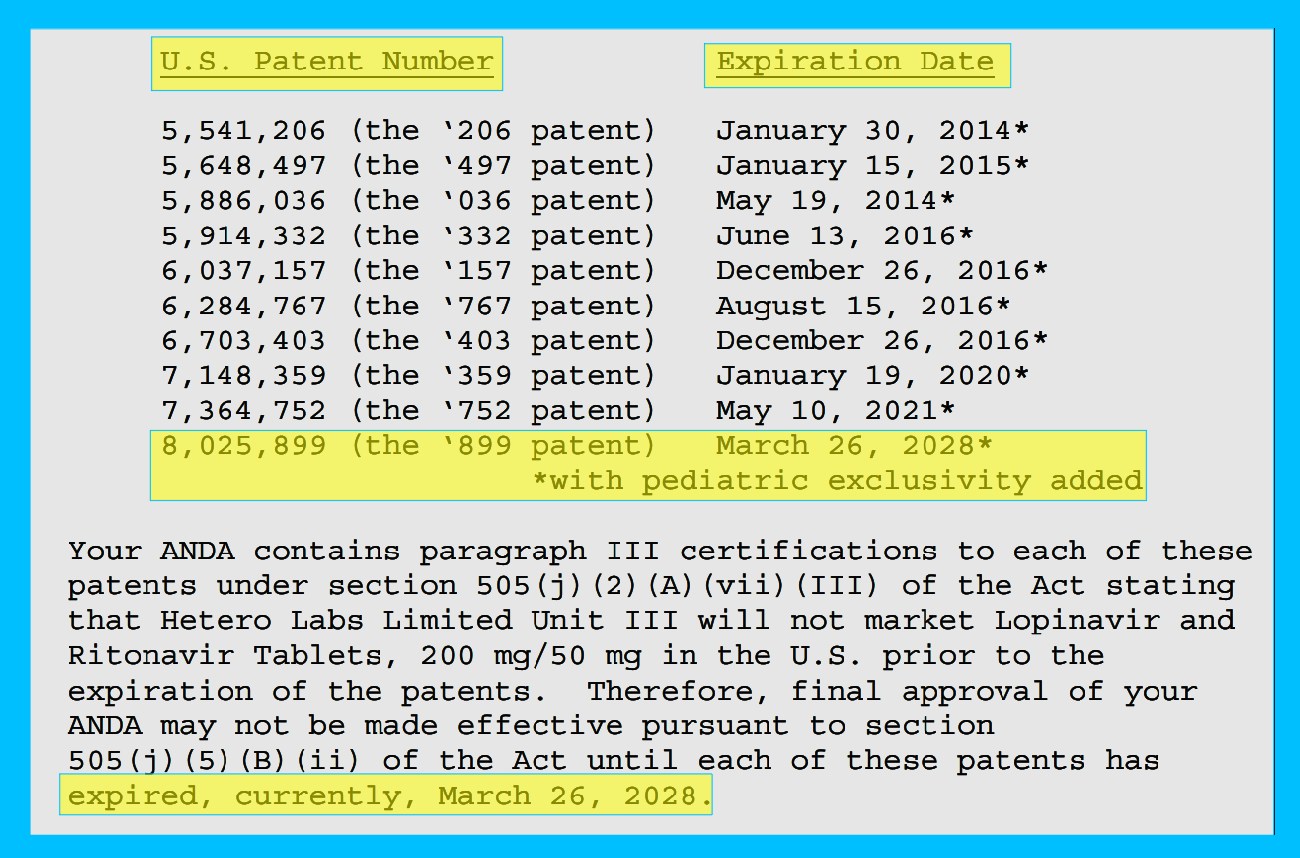
Because on May 10, 2021 they extended the patent on the drugs until March 26, 2028.
https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/091677s000ltr.pdf

